Abstract
Background: Covalent Bruton tyrosine kinase inhibitors (BTKi) have transformed the treatment landscape of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Despite the efficacy of covalent BTKi, treatment failure often occurs through development of resistance or intolerance. Pirtobrutinib, a highly selective, non-covalent (reversible) BTKi, inhibits both wildtype and C481-mutant BTK with equal low nM potency, and has favorable oral pharmacology that enables continuous BTK inhibition throughout the dosing interval regardless of intrinsic rate of BTK turnover. Pirtobrutinib is well tolerated and has demonstrated promising efficacy in patients (pts) with poor-prognosis B-cell malignancies following prior therapy, including prior covalent BTKi (Mato et al. Lancet, 2021). Here, we report updated CLL/SLL results from the BRUIN study (NCT03740529).
Methods: Pts with previously treated B-cell malignancies, including CLL/SLL, were eligible for treatment with pirtobrutinib monotherapy in either the dose escalation or expansion portion of the multicenter BRUIN study. Key endpoints included overall response rate (ORR) per 2018 iwCLL response criteria, progression-free survival (PFS), and safety. The response evaluable cohort consisted of all CLL/SLL pts enrolled to either phase 1 or 2 who had received a prior covalent BTKi containing regimen and had undergone their first response assessment or discontinued therapy. The safety cohort consisted of all pts with B-cell malignancies who received at least one dose of pirtobrutinib monotherapy (n=725). A data cut of 31 January 2022 was utilized.
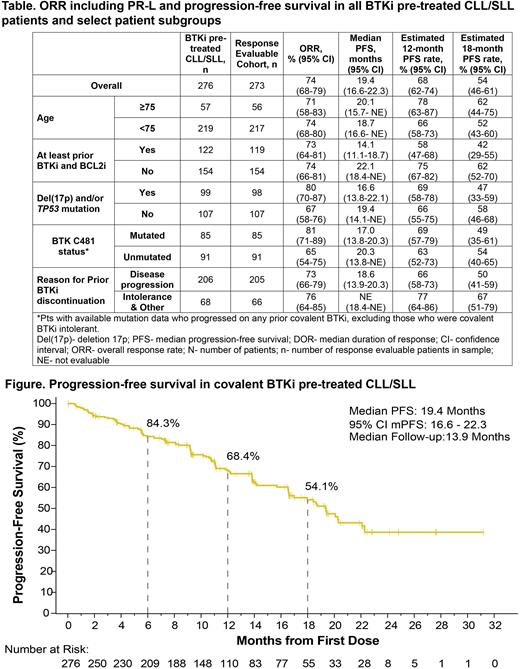
Results: Among the 276 pts with CLL/SLL who had received a prior BTKi, median age was 69 (range 36-88) years and the median number of prior therapies was 3 (range 1-11). Additional prior therapies included anti-CD20 antibody (89%), chemotherapy (80%), BCL2 inhibitor (44%), PI3K inhibitor (24%), CAR-T cell therapy (6%), and stem cell transplantation (2%). High-risk features were frequent: del(17p) in 29% (58/197), mutated TP53 in 40% (91/230), and unmutated IGHV in 85% (188/220). The majority of pts (n=206, 75%) discontinued prior BTKi therapy due to disease progression. Overall, 84% (n=232) received the recommended phase 2 dose of 200 mg once daily as starting dose. In this group of heavily pretreated relapsed/refractory CLL/SLL patients, including all with prior BTKi use, the ORR by investigator assessment was 74% (95% CI, 68-79) (Table) including 3 complete responses (1%), 174 partial responses (PR; 64%), 23 PRs with lymphocytosis (PR-L; 8%), and 1 nodular PR (<1%). At a median follow up time of 13.9 months, the median PFS was 19.4 months (95% CI, 16.6-22.3) (Figure). The 12-month and 18-month estimated PFS rates were 68% (95% CI, 62-74) and 54% (95% CI, 46-61), respectively. The ORR and PFS across various patient subgroups are depicted in the Table. In the safety cohort of all pirtobrutinib treated pts with B-cell malignancies (n=725), the most common TEAEs, regardless of attribution, were fatigue (26%, n=191), diarrhea (22%, n=160), and contusion (19%, n=138). The most frequent Grade ≥3 TEAE was neutropenia (20%, n=143). Low rates of Grade ≥3 TEAEs of hypertension (3%, n=20), hemorrhage (2%, n=16), and atrial fibrillation/flutter (1%, n=7) were observed. Overall, 15 (2%) pts discontinued due to a treatment-related AE.
Conclusion: In this updated analysis with additional pts and extended follow-up, pirtobrutinib continues to demonstrate promising and durable efficacy in heavily pre-treated R/R CLL/SLL pts who have been treated with a prior covalent BTKi, regardless of prior therapy, reason for prior BTKi discontinuation, age, high-risk TP53 mutations, C481 mutational status, and/or del(17p). Pirtobrutinib was well-tolerated with low-rates of discontinuation due to drug-related toxicity.
Disclosures
Mato:LOXO: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Nurix: Research Funding; Pfizer: Research Funding; BeiGene: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria; Genentech: Honoraria, Research Funding; Octopharma: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; TG Therapeutics, Inc: Honoraria, Research Funding; Pharmacyclics, LLC: Honoraria, Research Funding; DTRM Biopharma: Honoraria, Research Funding; Johnson & Johnson: Honoraria, Research Funding; Curio: Honoraria; Dava: Honoraria; BMS: Honoraria; Medscape: Honoraria; Acerta: Research Funding; PER: Honoraria; AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; PerView: Honoraria. Woyach:Loxo@Lilly: Research Funding; Karyopharm Therapeutics: Research Funding; Janssen: Consultancy; Schrodinger: Research Funding; Pharmacyclics: Consultancy; Newave: Consultancy; MorphoSys: Consultancy, Research Funding; BeiGene: Consultancy; ArQule: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Research Funding; Genentech: Consultancy. Brown:BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics: Research Funding; Abbvie, Acerta/Astra-Zeneca, BeiGene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, iOnctura, Janssen, MEI Pharma, Pharmacyclics: Consultancy. Ghia:BeiGene: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Lilly/Loxo: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Patel:AstraZenca, BMS, Celgene, Roche/Gen, Kite, Pharmacyclics/Janssen, TG: Speakers Bureau; Abbvie, ADC Therapeutics, AstraZeneca, Beigene, BMS, Caribou Biosciences, Celgene, Epizyme, Roche/Gen, Kite, MEI, Morphosys, Pharmacyclics/Janssen, TG, Trillium Therapeutics/Pfizer, Xencor: Consultancy; Adaptive Biotechnologies, AptevoTherapeutics, AstraZeneca, BMS, Celgene, CRISPR, Curis, Epizyme, Fate Therapeutics, Roche/Gen, Kite, MEI, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuticals, Trillium Therapeutics/Pfizer, Velos Bio, Xencor: Research Funding. Eyre:Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Loxo Oncology @ Lilly: Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView: Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Medscape: Speakers Bureau. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Lamanna:Octapharma: Research Funding; AstraZenenca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Loxo Oncology/Eli Lilly and Company: Research Funding; Mingsight: Research Funding; Genentech: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncternal: Research Funding; TG Therapeutics: Research Funding. Tam:Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria; LOXO: Honoraria; Beigene: Honoraria, Research Funding. Seymour:Celgene: Consultancy, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genor Biopharma: Membership on an entity's Board of Directors or advisory committees. Shah:Lilly Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy; Epizyme: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Kite Pharma: Consultancy; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Miltenyi Biotec: Consultancy, Research Funding. Coombs:CTI Biopharma: Current equity holder in publicly-traded company; TG Therapeutics: Honoraria; Novartis: Honoraria; Genentech: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria; Loxo/Lilly: Consultancy, Honoraria, Research Funding; MEI Pharma: Honoraria. Ujjani:Eli Lilly and Company: Consultancy, Research Funding; Loxo Oncology: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astara: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Genentech: Consultancy; Beigene: Consultancy; Incyte: Consultancy; Pharmacyclics: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding. Patel:ION Pharma: Other: Leadership; Exelixis: Speakers Bureau; Adaptive Biotechnologies: Honoraria; Bayer: Honoraria; Pharmacyclics: Honoraria; Blueprint Pharmaceuticals: Research Funding; Immunogen: Research Funding; Compugen: Research Funding; Accutar Biotech: Research Funding; Astellas: Research Funding; Adagene: Research Funding; Olema: Research Funding; Zymeworks: Research Funding; TeneoBio: Research Funding; Silicon Therapeutics: Research Funding; Samumed: Research Funding; Relay Therapeutics: Research Funding; Nurix: Research Funding; Novartis: Research Funding; NGM Biopharmaceuticals: Research Funding; Black Diamond Therapeutics: Research Funding; BioTheryX: Research Funding; Puretech: Research Funding; IgM Biosciences: Research Funding; MabSpace: Research Funding; Treadwell: Research Funding; Artios: Research Funding; ORIC: Research Funding; Vigeo: Research Funding; TopAlliance BioSciences Inc: Research Funding; Tesaro: Research Funding; Takeda: Research Funding; Taiho Pharmaceutical: Research Funding, Speakers Bureau; Syndax: Research Funding; Seven and Eight Biopharmaceuticals: Research Funding; Ribon Therapeutics: Research Funding; Prelude Therapeutics: Research Funding; Portola Pharmaceuticals: Research Funding; Placon: Research Funding; Pfizer: Honoraria, Research Funding; Moderna Therapeutics: Research Funding; Mirati Therapeutics: Research Funding; Millennium: Research Funding; Merck: Research Funding; Macrogenics: Research Funding; Lycera: Research Funding; LSK Biopartners: Research Funding; Loxo: Research Funding; Klus Pharma: Research Funding; Kymab: Research Funding; Janssen: Honoraria, Research Funding; Jacobio: Research Funding; Incyte: Research Funding; Ignyta: Research Funding; Hutchison MediPharma: Research Funding; Hengrui Therapeutics: Research Funding; H3 Biomedicine: Research Funding; GlaxoSmithKline: Research Funding; Gilead Sciences: Research Funding; Genentech/Roche: Honoraria, Research Funding, Speakers Bureau; FORMA Therapeutics: Research Funding; Evelo Therapeutics: Research Funding; EMD Serono: Research Funding; Eli Lilly and Company: Research Funding; Daiichi Sankyo: Research Funding; Cyteir Therapeutics: Research Funding; Clovis Oncology: Research Funding; CicloMed: Research Funding; Checkpoint Therapeutics: Research Funding; Celgene: Research Funding, Speakers Bureau; Boehringer Ingelheim: Research Funding; BioNTech AG: Research Funding; AstraZeneca: Research Funding; Aileron Therapeutics: Research Funding; Agenus: Research Funding; ADC Therapeutics: Research Funding; Acerta Pharma: Research Funding; Pfizer/EMD Serono: Consultancy; Pharmacyclics/Janssen: Consultancy. Fakhri:University of California San Francisco: Current Employment; Adaptive: Research Funding; BMC: Research Funding; Angiocrine: Research Funding; Seattle Genetics: Research Funding; Astrazeneca: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Lox/o Oncology/Eli Lilly and Company: Research Funding; CSL Behring: Consultancy. Cheah:Janssen: Consultancy, Honoraria; Merck Sharp & Dohme: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Alencar:Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; OncLive: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; SeaGen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo Oncology: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cohen:BeiGene: Consultancy, Research Funding; Genentech: Research Funding; Takeda: Research Funding; Lilly Oncology/Eli Lilly: Consultancy, Research Funding; Astrazeneca: Consultancy, Research Funding; Kite Pharma/Gilead: Consultancy; Aptitude Health: Consultancy; HutchMed: Consultancy, Research Funding; BMS/Celgene: Research Funding; Novartis: Research Funding; Janssen: Consultancy. Gerson:Loxo Oncology: Research Funding; Genentech: Consultancy; Abbvie: Consultancy. Flinn:Portola Pharmaceuticals: Research Funding; Verastem: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Servier Pharmaceuticals: Consultancy; Roche: Consultancy, Research Funding; Takeda: Consultancy; Forty Seven: Research Funding; IGM Biosciences: Research Funding; TG Therapeutics: Consultancy, Research Funding; Incyte: Research Funding; Century Therapeutics: Consultancy; Celgene: Research Funding; Gilead Sciences: Research Funding; Pfizer: Research Funding; Xencor: Consultancy; Biopath: Research Funding; Bristol Myers Squibb: Research Funding; CALIBR: Research Funding; CALGB: Research Funding; Fate Therapeutics: Research Funding; CTI Biopharma: Research Funding; City of Hope National Medical Center: Research Funding; Secura Bio: Consultancy; Myeloid Therapeutics: Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rhizen Pharmaceuticals: Research Funding; Genmab: Consultancy; Nurix Therapeutics: Consultancy, Research Funding; Triphase Research & Development Corp: Research Funding; Genentech: Consultancy, Research Funding; Seattle Genetics: Research Funding; Infinity Pharmaceuticals: Research Funding; Loxo@Lilly: Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; TCR2 Therapeutics: Research Funding; Janssen: Consultancy, Research Funding; Iksuda Therapeutics: Consultancy; Kite Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Unum Therapeutics: Research Funding; Hutchison MediPharma: Consultancy; Abbvie: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Agios: Research Funding; ArQule: Research Funding; Acerta Pharma: Research Funding; Constellation Pharmaceuticals: Research Funding; Curis: Research Funding; Forma Therapeutics: Research Funding; Merck: Research Funding; Trillium Therapeutics: Research Funding; Epizyme: Research Funding; Millenium Pharmaceuticals: Research Funding; Tessa Therapeutics: Research Funding; 2seventy bio: Research Funding. Ma:Juno: Research Funding; TG Therapeutics: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy; BeiGene: Consultancy, Research Funding, Speakers Bureau; Loxo: Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding. Jagadeesh:Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Debio pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Trillium Pharmaceuticals: Research Funding; ATARA Biotherapeutics: Research Funding; Seagen: Research Funding; Regeneron Pharmaceuticals, Inc.: Research Funding; AstraZeneca: Research Funding; Affimed: Membership on an entity's Board of Directors or advisory committees. Rhodes:Pharmacyclics: Consultancy, Research Funding; SeaGen: Consultancy; Abbive: Consultancy; TG Therapeutics: Consultancy; Oncternal: Research Funding; Janssen: Consultancy, Research Funding; Beigene: Consultancy; Morphosys: Consultancy; Genmab: Consultancy; Velosbios: Research Funding; Verastem: Consultancy; Genentech: Consultancy; Loxo Oncology: Research Funding; Epizyme: Research Funding. Zinzani:Secura Bio: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Balbas:Loxo Oncology/Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Nair:Loxo Oncology/Eli Lilly and Company: Current Employment. Abada:Loxo Oncology/Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Wang:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Wang:Loxo Oncology/Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Tsai:Loxo Oncology/Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Wierda:Oncternal Therapeutics: Research Funding; Cyclacel: Research Funding; Janssen: Research Funding; Kite Pharma: Research Funding; Miragen: Research Funding; Gilead Sciences: Research Funding; AstraZeneca/Acerta Pharma: Research Funding; Pharmacyclics, LLC an AbbVie Company: Research Funding; Genentech: Research Funding; AbbVie: Research Funding; Loxo Oncology/Lilly: Research Funding; Bristol Myers Squibb (June and Celgene): Research Funding; Sunesis: Research Funding; Xencor: Research Funding; GSK/Novartis: Research Funding. Jurczak:AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Celgene: Research Funding; Bayer: Research Funding; Mei Pharma: Research Funding; Lilly: Consultancy, Research Funding; Takeda: Research Funding; Roche: Consultancy, Research Funding; TG Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Sandoz: Consultancy, Research Funding; Merck: Research Funding; Beigene: Consultancy, Research Funding; Morphosys: Research Funding; Novo Nordisk: Research Funding.
OffLabel Disclosure:
We will be presenting data from the BRUIN trial. Pirtobrutinib is not approved yet.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal